News Story
Cheaper, Faster and Longer Lasting: What Magnesium Iodine Chemistry Can Offer

Image: First author and Chem/Bio PhD Candidate, Tao Gao.
Batteries – can you image how cumbersome the world would be without them? Without any moving parts, batteries convert chemical energy into electricity, making everyday life more expedient. Rechargeable lithium-ion batteries power our daily lives, from cell phones, to computers, and even electric vehicles. These batteries provide numerous benefits, but are not without disadvantages. Lengthy charging times and short overall battery life, for example, can be a burden.
Chunsheng Wang, a Professor within the UMD Department of Chemical and Bimolecular Engineering, and his team have developed an alternative to current technology. This new battery chemistry is based on the coupling of a magnesium cathode and an iodine anode. Magnesium batteries have the potential for much higher energy density – roughly 10 times current technology – however, development of rechargeable magnesium batteries has been slowed due to a lack of a compatible cathode. For readers who have forgotten their Chemistry 101 instruction, in an electrical current, you have positively charged electrodes (i.e. Anode) and negatively charged electrodes (i.e. Cathode), both of which are required to generate electricity in a battery. And a higher energy density translates into longer battery life, which could save consumers money in the long run.
“Our mission is to make rechargeable batteries that are cheaper, recharge faster and last longer than what is currently available to consumers,” said ChBE Ph.D. Candidate Tao Gao. “Research of magnesium batteries has been ongoing for the last decade, and one of the biggest challenges is decreasing the recharge time, to hours, rather than days.”
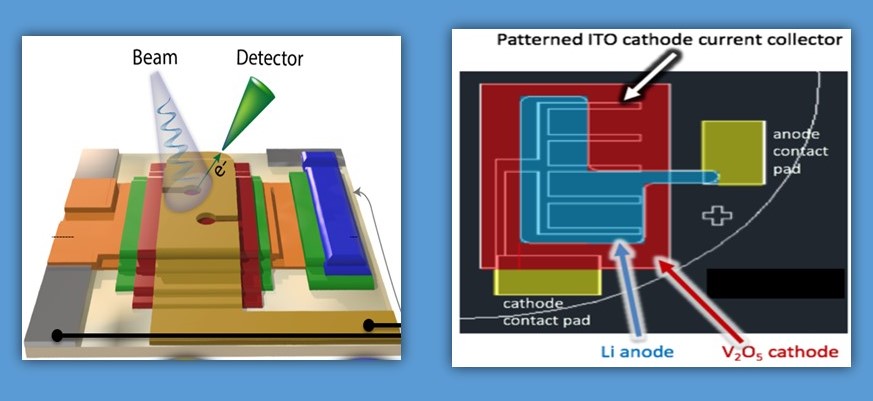
Dr. Wang’s research group approached this issue by combining iodine with magnesium. Iodine is soluble, and it becomes a solid after discharge, quite unlike the solid-state reaction in lithium-ion batteries. This two-phase, liquid-solid reaction is significantly faster than a pure solid reaction.
“In our research, we have demonstrated that the magnesium/iodine battery can be recharged within 5 min, “ said Gao. “This is very important for the direction of next generation battery chemistry because it dramatically decreases the charging time while significantly increasing the energy stored in the battery.” Gao and the rest of the team are currently studying the effects of temperature on this battery, to ensure that it can be used in all climates, under all conditions, regardless of application.
This groundbreaking research entitled, “High power rechargeable magnesium/iodine battery chemistry,” was published in Nature Communications on January 10, 2017. For additional information, or to read the paper in its entirety, please follow this link.
Published February 1, 2017